

Frontlines of Rare Disease Diagnoses: Interview with Genomics England's data scientists
Lifebit
%202.png?width=184&height=105&name=GEL_logo_RGB_Light%20(3)%202.png) We spoke to Dr. Dimitris Polychronopoulos, a Data Scientist, and Dr. Kristina Ibáñez Garikano, a Rare Disease Analyst, who are both part of Genomics England. Genomics England was set up by the UK Department of Health in order to orchestrate the 100,000 Genomes Project. Dimitris and Kristina are an incredible duo set to find ways of teasing out critical information from enormous data sets in order to deliver actionable insights to UK rare disease patients. Let’s explore how these superheroes go about fulfilling this tall order…
We spoke to Dr. Dimitris Polychronopoulos, a Data Scientist, and Dr. Kristina Ibáñez Garikano, a Rare Disease Analyst, who are both part of Genomics England. Genomics England was set up by the UK Department of Health in order to orchestrate the 100,000 Genomes Project. Dimitris and Kristina are an incredible duo set to find ways of teasing out critical information from enormous data sets in order to deliver actionable insights to UK rare disease patients. Let’s explore how these superheroes go about fulfilling this tall order…
The problem
The definition of a rare disease, is that it is in fact rare. The European Commission on Public Health defines a rare diseases as “life-threatening or chronically debilitating diseases which are of such low prevalence that special combined efforts are needed to address them”. More than 80% of the ~7,000 rare diseases, identified to date, are considered to be of genetic origin, and about 50% of patients are children1.
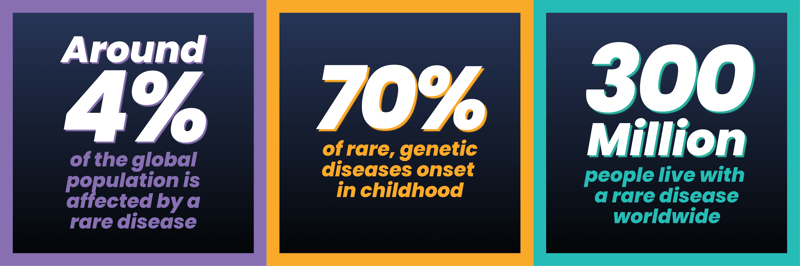
Although a vast majority of rare diseases have an identified genetic origin, traditional genetic testing only addresses a few genes at a time, thereby delaying the final diagnosis. More often than not, patients waste precious time and run out of options as clinicians are unable to provide answers solely through diagnostic testing. Therefore, powering rare disease diagnosis through genomic analysis makes sense in this context, which is exactly the strategy that Genomics England proposes in the 100,000 Genomes Project.
The solution to diagnosing rare diseases
The main objective of Genomics England’s 100,000 Genomes Project is to perform whole genome sequencing (WGS) on 100,000 NHS patients with rare diseases (or cancer) along with family members (genetic trios). This initiative is poised to greatly benefit patients by enabling new genomics-based discoveries and insights. But why might a patient need to get their whole genome sequenced?
WGS has allowed the scientific community to push the boundary of variant discovery in the context of rare diseases. By getting sequenced, patients can have their genomes analysed for specific variants that might prove to be the answer to their undiagnosable symptoms. The 100,000 Genomes Project is the first time such an in depth analysis of this magnitude has been performed on WGS data.
In order to achieve this, both Kristina and Dimitris employ state-of-the-art analysis techniques in order to deliver actionable insights for the NHS-registered clinicians they work in parallel with. Specifically, our superheroes analyse mendelian diseases in different structural families, perform quality analysis, identify different SNVs, Indels, CNVs, de novo variants, and short tandem repeats in these patients’ genomes. Furthermore, they benchmark variant callers and other tools used in-house, in order to optimise the analytical workflow to ensure correct diagnoses.
The importance of communication
Both Dimitris and Kristina agree that the diversity of the people working at Genomics England makes for a very interesting work environment, as you have a little bit of everything, including full stack developers, rare disease analysts, bioinformaticians, data scientists, software developers, project managers, among others. What’s more, researchers at Genomics England maintain close working relations with NHS-registered clinicians to allow for optimal communication and information transfer to patients.
However, it is a recognised challenge to speak the same ‘language’ among professionals from different specialisations. But being on the same page is of utmost importance in order to deliver timely diagnoses to rare disease patients. Communication is also enhanced through stand ups, and scrums in an Agile environment with sprints. For Kristina, this is a big change from academia, where researchers often times work in silos and are sometimes weeks or even months dealing with the same problem.
What Genomics England’s mission mean to these superheroes
For Kristina, joining Genomics England was both a personal challenge and a chance for her to make a tangible difference in the world of rare diseases. The 100,000 Genomes Project is a challenging programme because it is the first of its kind, specifically due to the fact that the cost of sequencing patients’ whole genomes is prohibitive on a large scale. And because genomic data on rare disease patients is so scarce, Kristina analogises her work at Genomics England as “having to Google search a question that nobody in the world has ever asked before”.
Dimitris also adds that at Genomics England, they are significantly contributing to the rare disease field by directly helping families with actionable findings about their genomes. Scientists like Kristina and Dimitris are contributing to the enrichment of scientific knowledge and favouring basic research by identifying previously unknown genetic variants. Genomics England combines diagnostics with basic science, thereby resulting in an interesting mix between industry and academia.
What’s more, the 100,000 Genomes Project is setting the stage for the new normal in the context of NHS, as it has been recently announced that it is systematically offering WGS to patients whom it would benefit.
Keep on trudging!
About Dr. Dimitris Polychronopoulos and Dr. Kristina Ibáñez Garikano
 Dimitris first started his studies in Molecular Biology, but after two years at the bench, he decided to make the switch to bioinformatics for his Masters. He continued on to his PhD at the University of Athens, and as a postdoc at Imperial College, where he focused his studies on computational regulatory genomics and non-coding elements. In stark contrast to Dimitris, Kristina actually started out her studies as a computer scientist. During her PhD at the CNIO in Madrid, she used machine learning techniques and artificial intelligence to analyse microarray data from different cancer types and neurodegenerative disorders. During her postdoc, Kristina worked on analysing SNVs, Indels from customised panel genes as well as exomes in INGEMM (Hospital Universitario La Paz, Madrid). In particular, she implemented a strategy to detect CNVs from targeted gene panels that is currently in clinical production
Dimitris first started his studies in Molecular Biology, but after two years at the bench, he decided to make the switch to bioinformatics for his Masters. He continued on to his PhD at the University of Athens, and as a postdoc at Imperial College, where he focused his studies on computational regulatory genomics and non-coding elements. In stark contrast to Dimitris, Kristina actually started out her studies as a computer scientist. During her PhD at the CNIO in Madrid, she used machine learning techniques and artificial intelligence to analyse microarray data from different cancer types and neurodegenerative disorders. During her postdoc, Kristina worked on analysing SNVs, Indels from customised panel genes as well as exomes in INGEMM (Hospital Universitario La Paz, Madrid). In particular, she implemented a strategy to detect CNVs from targeted gene panels that is currently in clinical production
About Lifebit
At Lifebit, we develop secure federated data analysis solutions for clients including Genomics England, NIHR Cambridge Biomedical Research Centre, Danish National Genome Centre and Boehringer Ingelheim to help researchers turn data into discoveries.
Interested in learning more about Lifebit’s federated data solution for genomics research?
References
- Boycott, Kym M., et al. “Rare-disease genetics in the era of next-generation sequencing: discovery to translation.” Nature Reviews Genetics 14.10 (2013): 681.
- Carss, Keren J., et al. “Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease.” The American Journal of Human Genetics 100.1 (2017): 75-90.
Featured news and events
2025-03-26 11:17:46
2025-03-14 15:45:18
2025-03-05 12:49:53
2025-02-27 10:00:00

2025-02-19 13:30:24
2025-01-30 12:47:38

.png)